 |
 |
6/04/2010 |
What is Electrophoretic Deposition?Electrophoretic Deposition (EPD) is a particle forming process most often used for form coatings on a conductive surface, but which also can be used to create free standing objects or as a step in a particulate printing process. The result of EPD is a particle compact which must be either sintered to full density or infiltrated with a binder to achieve any significant strength. EPD is superficially a very straightforward process that can be defined simply as follows: |
|
|
Analytic
Definition:
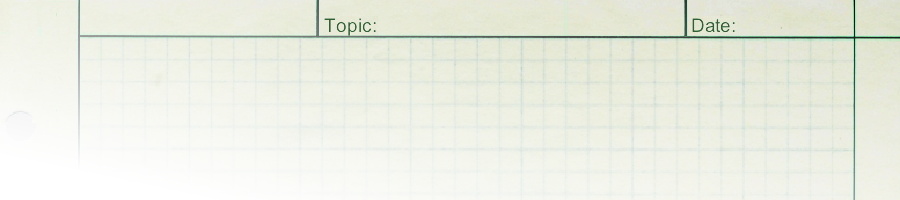
1. Form a stable suspension of charged particles in a solvent. 2. Create a voltage field in the solvent which will induce the electrophoretic motion of the particles toward the oppositely charged electrode. 3. When the particles arrive at the electrode they deposit to form a rigid adherent coating. |
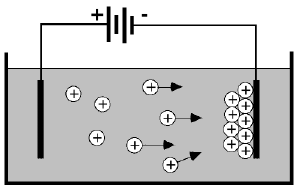 Analytic concept of EPD |
|
The above definition is based on a logical statement of how EPD is expected to work. It is the definition which gives the process its name, since it requires both electrophoresis and deposition. However, although it is never explicitly stated, there is a practical definition of EPD that has been used consistently from the very first experiments on this process. |
|
|
Practical
definition:
1. Place two electrodes into a mixture of solvent and particles. 2. Apply a voltage/current between the electrodes for a set period of time. 3. If after removal one of the electrodes has a coating of particles, then EPD has occurred. |
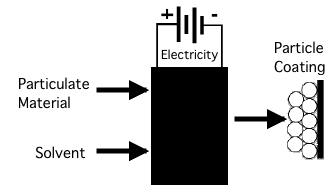 Practically EPD is often a black box process. |
|
The difficulty arises when people assume that if a process is EPD according to the practical definition then it must also be EPD by the analytic definition. The analytic definition, however, is much more restrictive than the practical. It requires both electrophoresis and deposition of particles in suspension with the implicit requirement that the particles be fully dispersed, since the particles need to be able to move independently to move by electrophoresis. The practical definition does not require that the particles deposit or that they move at all, whether by electrophoresis or any other mechanism. The only requirement of the practical definition is that the application of a voltage/current result in some sort of change that allows an electrode to be removed from the particle/solvent mix with a layer of particles. (If the electrode can be dipped into the particulate mixture and removed with a coating of particles without the voltage/current, this is called 'Dip Coating' and is well defined as a different process.) Two ExamplesTwo hypothetical examples can help to illustrate the situation. Consider first a 40 vol.% suspension of silica particles in water that are fully dispersed and electrostatically stabilized with a negative surface charge at a slightly basic pH and a significant concentration of a neutral salt. The viscosity will only be 4-5x that of water so there will be no significant dip coating. Assuming porous electrodes so that electrolysis gases can escape, a voltage is applied which is the sum of the 1.25 V that will be concentrated in the boundary layers to allow the electrolysis reaction to go forward and a voltage dividing out to 1 V/cm to drive current across the cell. Now as water is electrolyzed at the electrodes the solution will become more basic at the cathode and more acidic at the anode. In this case it is clear that deposition will occur. The motion of the acidic electrochemical boundary layer into the suspension will destabilize the silica particles, allowing them to come into contact to form a deposition. However, no detectable level of electrophoretic motion will occur. The deposition will be at the same density as the particles in the suspension. It is during drying that they will be compacted to better than 50 vol.%. Because of the steady progression of the electrochemical boundary layer, the weight of the deposition will be linear with time. A novice experimenter, having performed EPD according to the practical definition will return to the analytic definition and conclude that the particles have moved electrophoretically toward the anode where they deposit as they arrive. A more extreme example is an experimenter who attempts to deposit particles from a polymerically stabilized slurry in a MEK-tolulene mixture. To test whether this is possible the experimenter uses freshly polished brass electrodes. These electrodes are not wetted by the solvent, therefore if they are dipped without applying a current, they can be removed cleanly. However, when a current is run between the electrodes in the slurry, the positive electrode develops a thin anodic oxide coating, losing residual wax from the polishing compound in the process. The anode now wets the solvent very well and when removed it will entrain a layer of solvent and particles (i.e. it will now dip coat). This dip coated laver can now be dried to form a thin, even, adherent particle coating. The experimenter who has set out to perform EPD according to the analytic definition above will now happily report that negatively charged particles have electrophoretically migrated to and deposited on the anode. The first example was a process that involved deposition without electrophoresis, the second example is of a process without either electrophoresis or deposition. There are other examples of electrophoresis without deposition, while there are even more examples of particle consolidation by processes other than electrophoresis, with or without deposition. Choosing a DefinitionSo we are faced with a situation, there are ways that a particle coating can be produced by a combination of a colloid and a voltage/current that may not involve either electrophoresis or deposition or either. So, should we demand that a process demonstrate both electrophoresis and deposition before it can be called EPD? For better or worse, such attempts to maintain the purity of a definition are usually doomed to failure. Moreover, attempting to do this would create two significant problems. First, there is the question of how to deal with an already large number of publications which group a large variety of more or less well understood processes under the heading of EPD. The second is how to deal with researchers who, having having applied a voltage/current to a colloid and generated a coating, have completely exhausted their store of curiosity and do not care whether the particles have moved or how, or what the internal structure of their coating is before it is dried. The far simpler course of action is to accept the practical definition of EPD, but to make explicit that EPD does not necessarily involve either electrophoresis or deposition and may involve a variety of other processes. Just as deposition was shown not to be a single process (here) EPD needs to be broken down into components to show how colloids and electric currents can be combined to generate a layer of particles on an electrode. Components of EPDSo what are the component processes of EPD? Electrophoresis of a single particle in suspension is a well analyzed process, the mechanisms of deposition have been discussed elsewhere (here), which leaves two key topics which have not received appropriate attention in discussions of EPD. The first topic is the mechanical behavior of particle suspensions between Newtonian liquid and brittle solid. These particle solvent mixtures are generally pseudoplastic with a range of elastic moduli, yield stresses and plastic flow viscosities. The rate of change of these properties as a function of particle volume fraction is an important part of the equation for determining the density that can be achieved during EPD. (and vitally its behavior during drying, i.e. cracking, curling and consolidation.) The second topic is the flip side of the mechanical response of the particles, it is the electrocapillary forces acting on those particles. This includes electrocapillary pumping between the particles, electrostatic forces arising from conductivity gradients, and osmotic pressures resulting from electrochemical concentration gradients. Analyzing these forces and the corresponding mechanical responses is a long term project, but by acknowledging that they exist it is now possible to breakdown the various processes that fall under our broadened heading of EPD. True electrophoretic deposition — This is a process that begins with a fully dispersed particulate and proceeds to a fully deposited body of particles. Naming this "true" EPD is not meant to be a value judgement. For many applications this can be an inferior process to others listed here. "True" is only intended to indicate that this process involves both electrophoresis and deposition, although without excluding the possibility of an electrocapillary consolidation in between. Electrocasting — This name is created by analogy to slip or tape casting. This process begins with a slip or slurry which may or may not be fully dispersed, which is liquid like (i.e. having a very low shear yield strength), but where the particles cannot be treated as moving independently by electrophoresis. The process ends with a colloid which has not changed in any significant way other than an increased volume fraction of particles. It is this densification that changes the colloid from a liquid-like state to densified state that has a high enough shear yield strength that it can be removed from the deposition cell and handled as if it were a solid. Electrophoretic Consolidation — This is the same as electrocasting above, but begins with a stable, low density dispersion of particles that move electrophoretically. Electrocasting with deposition — This is the same as electrocasting, but with an added deposition step. The particles are consolidated at the electrode to the point where the cannot move relative to one another, after which a deposition process takes place that turn the particle compact into a rigid body which cannot easily be rinsed away. Particulate Deposition — This is where there is deposition of particles but without electrophoresis or other particle motion. Practically this means putting the electrode into a dense particulate slip, running a current through the electrode to produce a uniform, adherent layer of particles on the electrode that can then be removed and rinsed, but are at density no higher than in the liquid slip. Electrophoretic Consolidation — This is the case where there is electrophoresis, but no deposition. The particles are not deposited but form a dense enough colloid that this packed layer of particles can be removed from the deposition device with the deposition electrode. What is EPD?For practical purposes, EPD will be any process that combines particles, solvent and electricity to generate a coating of particles on an electrode. By hypothesizing all of the means by which this can occur, without necessarily including 'electrophoresis' or 'deposition', we can come up with a set of categories which begin to make sense of the seemingly irreconcilable variety of EPD processes present in the literature. |
|

 |
E-mail: vtweb@vtscience.com |